As the number of coronavirus cases inches closer to the 14 million mark and more than 5.9 lakh deaths globally, scientists and medical experts are racing against time to develop a vaccine to curb the spread of the virus.

According to a document prepared by the World Health Organisation, as of 15th July, 23 vaccine candidates have entered clinical evaluation stage and 140 vaccine candidates are in the preclinical evaluation stage globally.
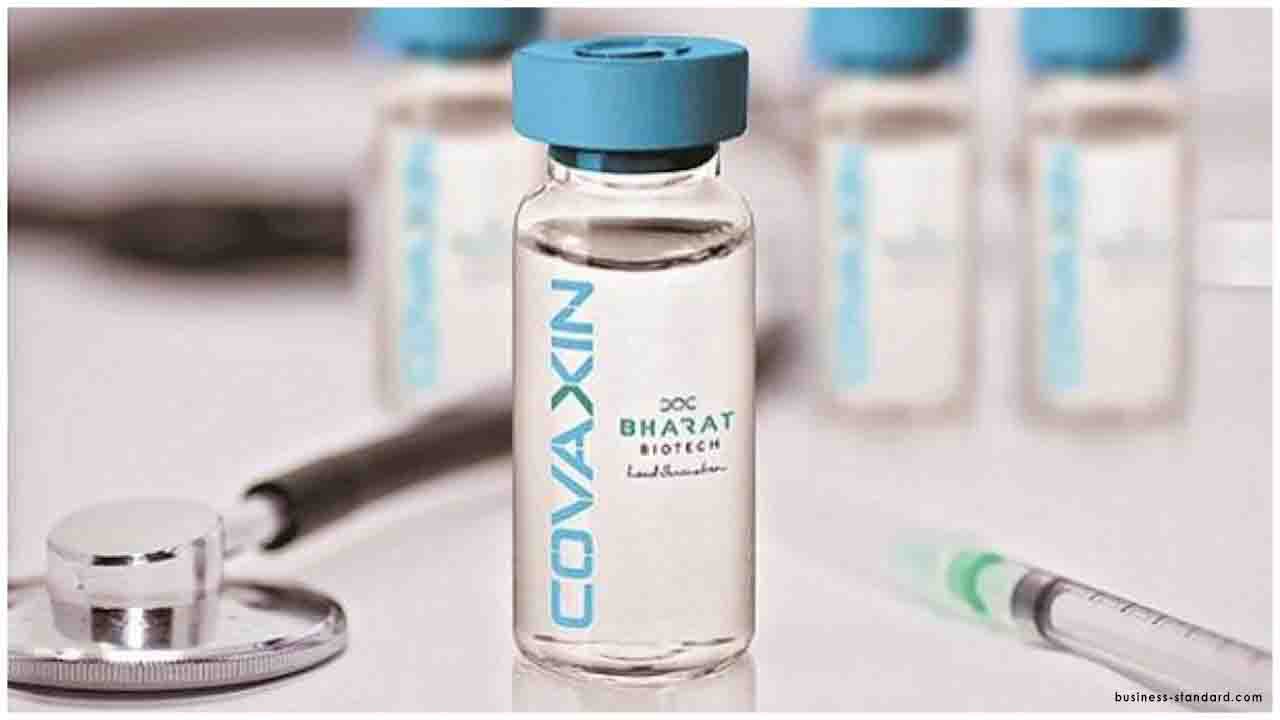
In India, two vaccine candidates have been approved and have entered clinical trials stage. Covaxin by Hyderabad based Bharat Biotech and ZyCoV-D by Ahmedabad based Zydus Cadila are preparing to conduct clinical tests.
Another vaccine, which is undertrials, developed by the researchers at the Jenner Institute of Oxford University has shown promising results after the first phase of clinical trials.
The medical journal ‘The Lancet’ has confirmed that it would be publishing early-stage human trials data from the Oxford team.
The Daily Telegraph reported a senior source from the trials saying that the Blood samples taken from a group of UK volunteers given a dose of the vaccine showed that it stimulated the body to produce both antibodies and “killer T-cells”.
Different studies have suggested that antibodies may disappear in months while T-cells remain in circulation for years. The results do not yet prove whether the vaccine provides long-lasting immunity from the virus.
According to a report published in The Telegraph, Professor Adrian Hill, the director of the Jenner Institute, told a webinar of the Spanish Society of Rheumatology that the “best scenario” would see results from clinical trials in August and September and Vaccine rollout in October.
The vaccine has been licenced to AstraZeneca. The chief scientist at the World Health Organization said in June that AstraZeneca’s vaccine candidate was probably the most advanced in terms of development.
AstraZeneca has signed agreements with governments around the globe to supply the vaccine if the results prove effective. The vaccine is currently in a combined Phase II/III trial in UK and Phase III trials in Brazil and South Africa.