Scientists at the National Institute of Virology in Pune have successfully developed a rapid test kit that can detect antibodies that develop in response to SARS-CoV-2.
Union Health Minister Harsh Vardhan tweeted about the same on Sunday.
National Institute of Virology, Pune has successfully developed the 1st indigenous anti-SARS-CoV-2 human IgG ELISA test kit for antibody detection of #COVID19 .
— Dr Harsh Vardhan (@drharshvardhan) May 10, 2020
This robust test will play a critical role in surveillance of proportion of population exposed to #SARSCoV2 infection pic.twitter.com/pEJdM6MOX6
The test is based on Enzyme-Linked Immunosorbent Assay (ELISA), which is designed particularly for screening large numbers of people at a time, making them suitable for detecting the spread of the disease in a large population within a short span of time. ELISA has been used previously to detect antibodies to HIV, syphilis and Zika virus.

The test kit has been validated at two sites in Mumbai & has high sensitivity & accuracy and has the advantage of testing 90 samples together in a single run of 2.5 hours so that healthcare professionals can proceed quickly with necessary next steps on their patients’ triage paths.
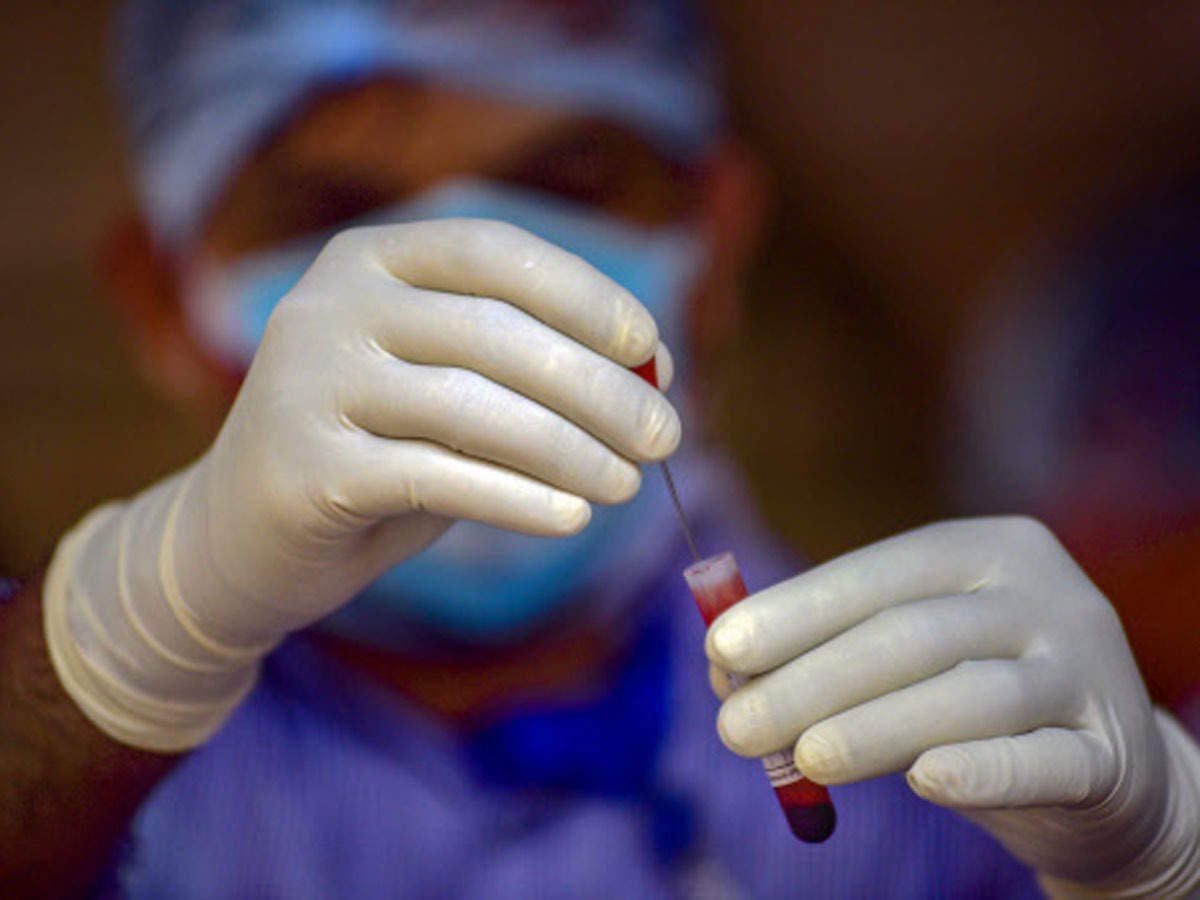
The ELISA test involves adding a specific antigen, a molecule that is present on the surface of a pathogen to the blood sample. If the blood contains antibodies to the antigen, the two will bind together. Observing this reaction allows scientists to determine if the blood contains antibodies.
The kit is believed to detect the presence of antibodies in the bloodstream of a person who may have been exposed to SARS-Cov-2. The antibodies are produced in response to an infection and their presence indicates that the person has been exposed to the novel coronavirus at some point.
Antibody tests are not used to detect Covid-19 infection in humans. Rather, they help detect the presence of antibodies that attempt to fight off the virus. The test helps estimate what percentage of the population came in contact with the virus and how far it spread.
NIV’s ELISA test can detect immunoglobulin G (IgG) antibodies, which are the most common type of antibodies found in blood circulation once the immune response against an infection kicks in. However, it may take two to three weeks to develop these antibodies in response to a pathogen.

This means that if a person is tested immediately after coming in contact with the novel coronavirus, the test may not reflect accurate results.
The technology has been transferred to Indian pharmaceutical company Zydus Cadila, which will be responsible for the mass production of the test.

















