India’s topmost medical research body Indian Council of Medical Research (ICMR) said on Friday that it is attempting to launch the world’s first Covid-19 vaccine, Covaxin on August 15.

The news has kept the people divided. While it comes across as good news, it has confused a lot of scientists and experts considering the long procedure to be followed before a vaccine is put out there as a cure.
The August 15 target for launching Covaxin, is being described as a fast-track effort. This is because the development of a vaccine is not just a long but also an uncertain process. It is also expensive, with funding playing an important role.

The vaccine has been jointly developed by ICMR and Hyderabad-based Bharat Biotech (BBIL). In an interview to New Indian Express on Wednesday, Bharat Biotech chairman and managing director Krishna Ella said if clinical trials of Covaxin met safety and efficacy standards, the vaccine could be available for mass use by early 2021.
In fact, BBIL, in its filing to the Clinical Trial Registry of India (CTRI) has stated that follow-ups for the clinical trial will be conducted on the 14th, 28th, 104th and 194th day, which clearly means a timeline of beyond 6 months. Additionally, the company lists the date of enrolment for the first phase of clinical trials from July 13, almost a week after the ICMR’s deadline for enrolment.
The vaccine will only be available for use after the clinical trials are over. Clinical trials include a small group of people tightly monitored inoculation among sets of volunteers to test whether a vaccine is safe and works, a process that on average has taken over 10 years.
There is no fixed period but the process can typically run into decades without any concrete results. For instance, after three decades of research, the vaccine for HIV is still in phase III of clinical trials. One of the fastest developed vaccines is the one used for mumps, which received approval in four years after trials began in 1963.
In a pandemic, this timeline can be compressed, but most experts believe that is still likely to take 12-18 months making India’s plans on Covaxin scientifically implausible. It’s not just the unreasonable deadline which critics believe is the problem but also that it would affect safety, efficacy and quality.
It is also believed just recruitment of participants in itself can take months even if we try to expedite everything like we are doing for Covid-19. Each of the phases, one, two and three, should take at least a few months as it is not just one centre but multiple centres that are involved in conducting trials to determine safety and efficacy of a vaccine.

Some experts have also said that in one month, it is possible to determine immediate safety, tolerability and immunogenicity of a candidate vaccine. This is sufficient to determine whether the approach has some promise or not. However, promising at this stage means just that, not effectiveness in preventing infections.
Covaxin is yet to enter the clinical trials phase, a stage that 18 candidates across the world have already raced to. The earliest any of these are expected to determine safety and efficacy is not until the second quarter of next year.
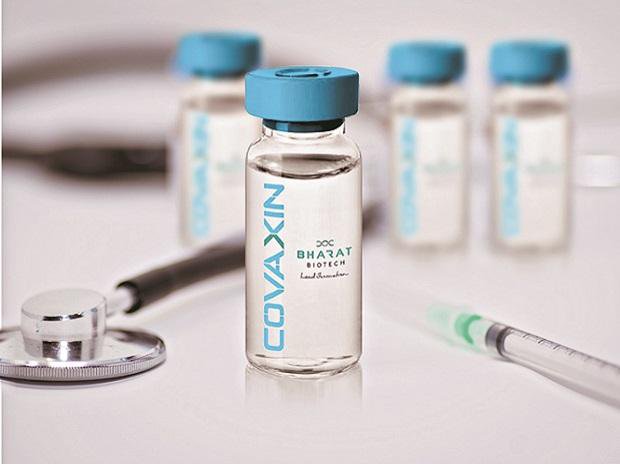
ICMR’s news of launching the vaccine also surprised experts because data about the vaccine’s preclinical performance has not been made public yet. Hence, it seems to be very ambitious and probably unprecedented.