India’s apex health research body, the Indian Council of Medical Research (ICMR) on 12th April uploaded proposals, inviting health institutions, hospitals to participate in two randomised controlled trials, convalescent plasma therapy and plasma exchange therapy.
Within a few days of the proposal, India has already got onto implementing the trials. The doctors have turned to the 1,600 people in India who have recovered from the infection to test their antibodies as a potential cure for those fighting the disease.

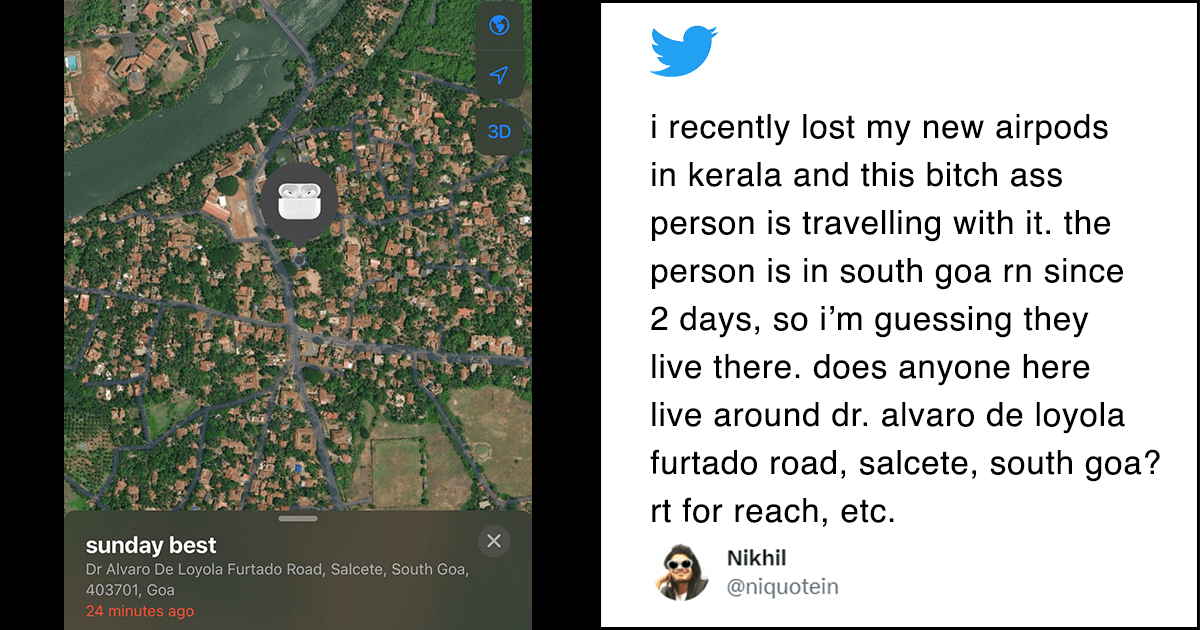
Convalescent plasma therapy involves injecting patients with plasma from people who have recovered from the infection, and whose bodies have, therefore, generated the antibodies required to fight the virus.
When the virus enters the human body, the immune system creates an antibody to fight it. This antibody remains in a person even after the virus has cleared out of their system. In the absence of other drugs, experts say that using these antibodies from recovered patients can offer a reprieve to severely ill Covid-19 patients.

The governments and experts believe to provide the therapy only to those who are in a critical condition. People with a respiratory rate higher than 30 breaths per minute as compared to a normal of 18-20 breaths per minute, have oxygen saturation of less than 90% or have infiltrates like pus in the lungs.
The criteria for the donor includes people with no comorbidities like diabetes, hypertension, or heart disease, needs to be less than 60 years of age and someone who has recovered from the infection would be selected. For the treatment, a plasmapheresis machine is used to derive plasma from the blood, which is then administered to the patients with severe infection.

Plasma therapy in India has been in use for three categories of ailments, one is viral infection such as hepatitis or even chicken pox that can be severe in immuno-compromised patients, it is done for autoimmune disorders like Rheumatoid arthritis and type 1 diabetes, and three, conditions like haemophilia in which the people receive proteins other than antibodies.
A recently published report on a trial in China showed an improvement in the clinical conditions of 10 people who received the therapy. All symptoms in the 10 patients, especially fever, cough, shortness of breath, and chest pain, disappeared or largely improved within 1 day to 3 day upon Convalescent Plasma transfusion. Even a person in Delhi was removed from the ventilator support after the plasma therapy.
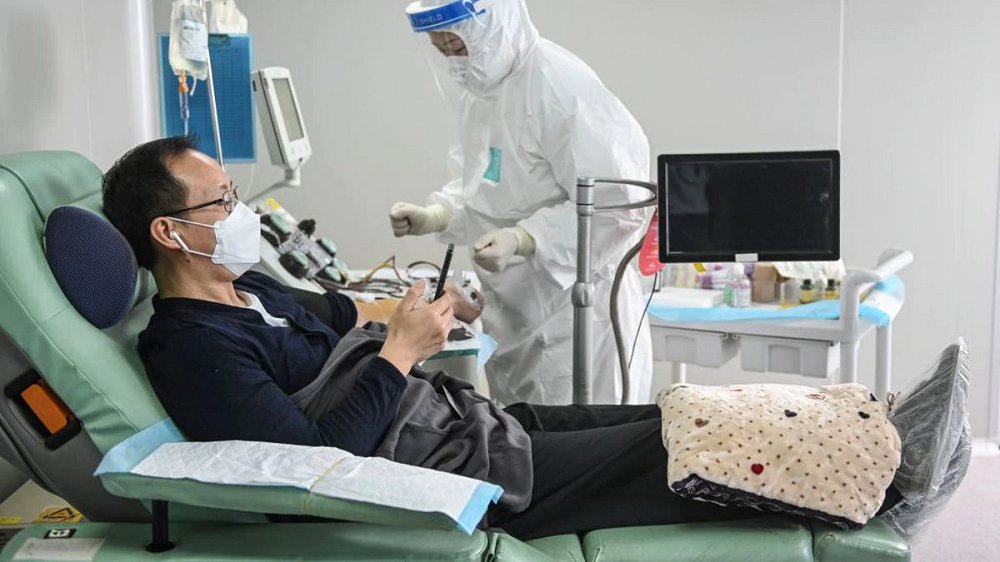
The important question that arises is that if the therapy was known even before why hasn’t it been brought to use yet. Well, the simple answer is because until now India didn’t have a pool of Covid-19 recovered patients. Now, when India has a pool of 1,600 people, the therapy seems closer to being implemented.
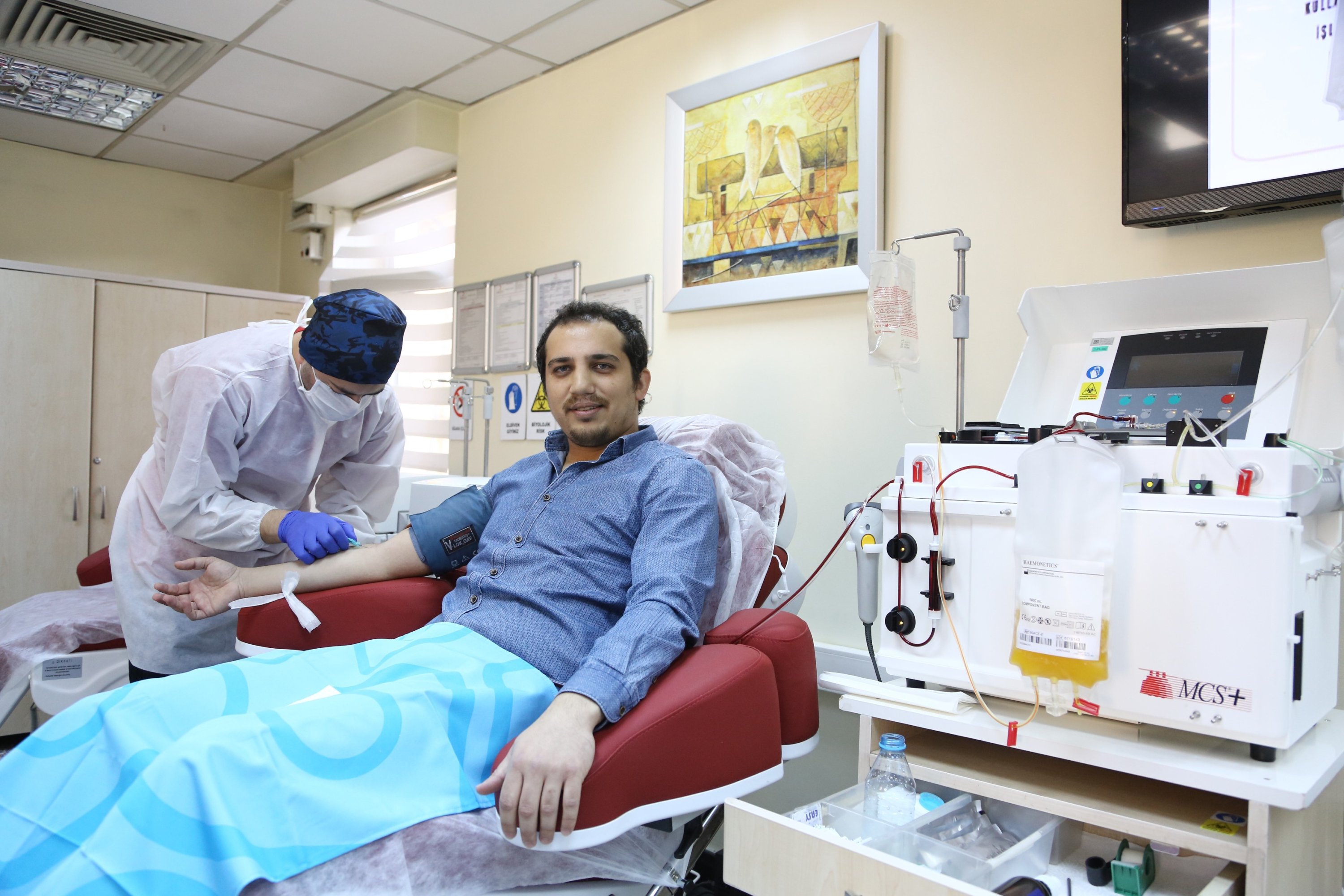
Kerala was the first state in India to have announced the treatment through the therapy but is now waiting for the permissions to start the procedure.
As of now, the Institute of Liver and Biliary Sciences, an autonomous Delhi government hospital, has already received approval to conduct the trials while the private hospital Max awaits approvals to launch the trials. The approvals are being given to hospitals that conform with the ICMR protocols so that uniform and comparable data is generated from across centres.
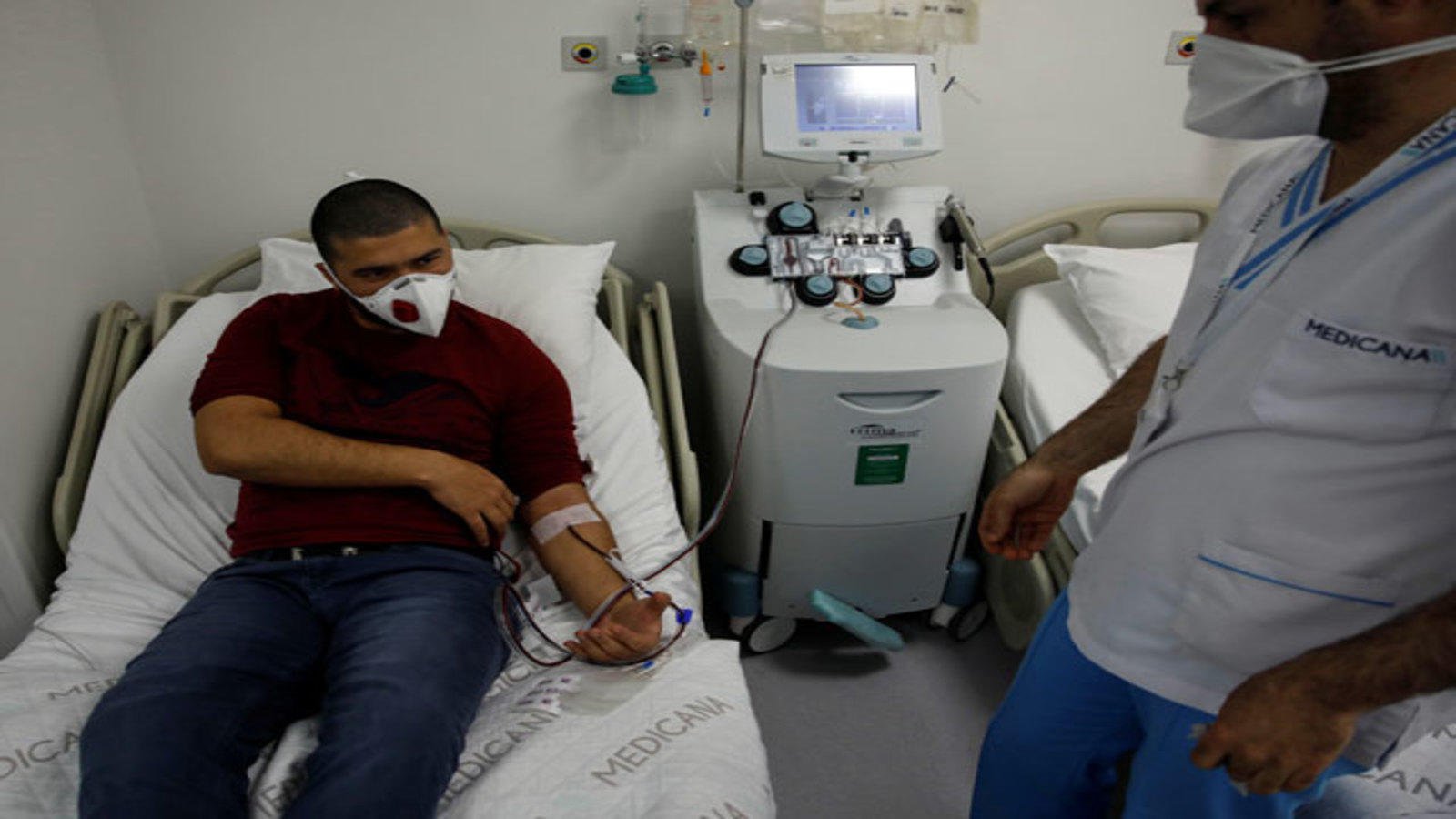
So what are the protocols that the hospital needs to abide by?
The hospitals need to develop a trial protocol that has to be approved by the Institutes Ethics Committee. It is important that the hospitals are registered with the Clinical Trial Registry of India and the country’s apex drug regulator- Central Drugs Standard Control Organisation (CDSCO).